Perfusion bioreactors
Perfusion bioreactors are used for cells that require a surface on which to grow. This (usually static) cell substrate is gently perfused with aqueous fluid containing nutrients and other media, such as differentiating agents, to ensure all cells are sufficiently nourished and have the desired properties. These differentiating agents are used to convert stem cells into cells for a particular tissue, such as bone or muscle.
A wide array of perfusion bioreactor setups have been explored to date, ranging from commercial-scale and larger lab-scale processes, to one-off bespoke lab equipment.
How CFD can optimise perfusion bioreactors
CFD is relatively limited in its ability to support the development of perfusion bioreactors, however studies have demonstrated its potential in optimising bioreactor designs and cell culture processes, in order to augment cell proliferation.
For example, factors such as fluid shear and pressure gradients that may cause cell damage can be quantified and minimised using CFD. Additionally, CFD can be used to examine and optimise the perfusion rate of fresh nutrients and other media across different locations on the reactor. This can help accelerate cell and gene development programmes by informing which experiments are likely to be most successful.
From a fluid dynamics perspective, CFD models are typically straightforward to execute. CFD is an ideal tool to understand the flow within complex 3D geometries including the impact of small manufacturing defects. To date, it has been successfully applied to commercial cell processing equipment and bespoke lab setups, including complex 3D scaffolds that mimic biological tissue structures.
Case study – using CFD to troubleshoot cell cube reactors
The following case study illustrates how CFD can be used to understand and resolve issues with poor cell growth within a bioreactor. The Corning® CellCube® Culture System is a commercially available bioreactor that facilitates cell growth on diamond-shaped parallel plates, which are perfused by a diverging-converging flow. During early evaluations of the device, identified on these plates, including regions of poor cell growth. A team of researchers conducted investigatory CFD simulations to identify the cause, using flow patterns and other CFD data to explain the majority of the uneven growth patterns.
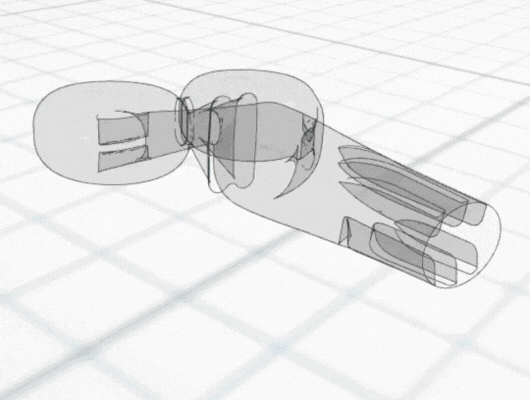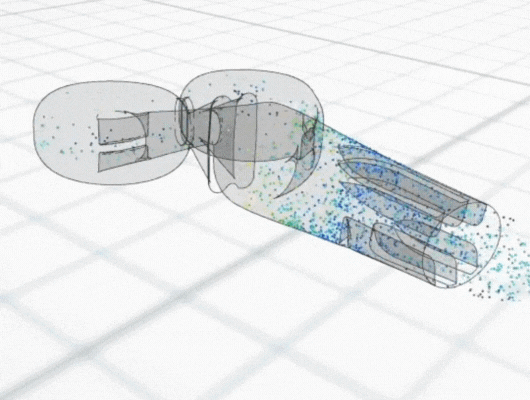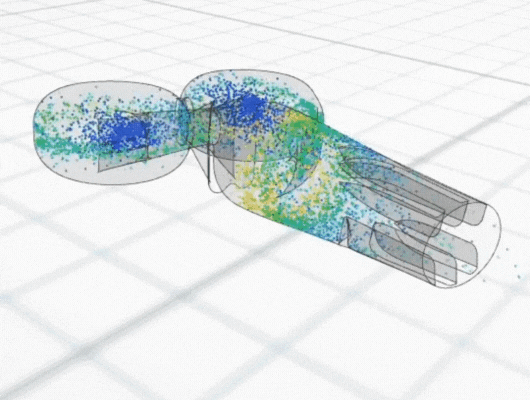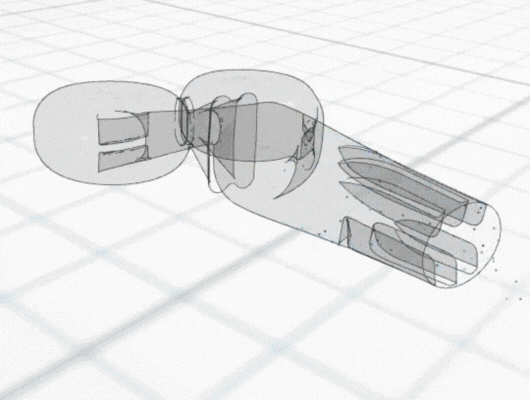
Using particle-tracking analysis, an in-built CFD tool, to model the trajectories of the cells within the equipment, the team discovered low cell growth at the edges of the front plate could be attributed to initial cell seeding not extending to the plate edges. Meanwhile, regions of poor cell growth at the inlet nozzles and central portion of the back plate were attributed to high shear rates leading to cell detachment.
The CFD analysis led to proposed improvements to eliminate the issues with poor cell growth, including eliminating plate bowing and identifying alternative cell seeding approaches.
Case study – scaffold reactor selection
As mentioned, perfusion reactors can be used to build complex 3D scaffolds that mimic biological tissue structures. In one study, researchers compared the flow patterns through a scaffold cell-culture substrate in two reactor designs, in order to optimise the media perfusion rates to different regions of the scaffold. The porous scaffold provided a three-dimensional representation of the bone microstructure. This environment was designed to engineer bone tissue from mesenchymal stem cells.
Using CFD models, analysts identified notably different flow distribution behaviour between the two reactor designs. One design resulted in much reduced fluid shear and more even nutrient perfusion throughout the scaffold, compared to the other. These modelled findings were confirmed by higher cell growth rates in the improved reactor design.
Overall, CFD proved instrumental in understanding how to optimise shear stress for cell growth and osteogenic differentiation.