By leveraging analytical tools and methodologies, engineers can make informed decisions, iterate rapidly, and reduce the reliance on physical prototyping, ultimately accelerating the development timeline. One of the key tools within engineering analysis is mathematical modelling.
Math modelling – early-stage analysis
A mathematical model is a simplified representation of a system using mathematical equations and techniques. It is useful to apply throughout your development, however, can be particularly useful in the early stages for determining technical feasibility.
One of the key benefits of a mathematical model is the ability to understand complex systems without having to physically develop and test them. By formulating mathematical equations and relationships, you can gain insights into the behaviour, interactions and dynamics of complex phenomena. This can be used to predict how certain components will interact with each other for example, or to estimate the probability of failure or certain events occurring.
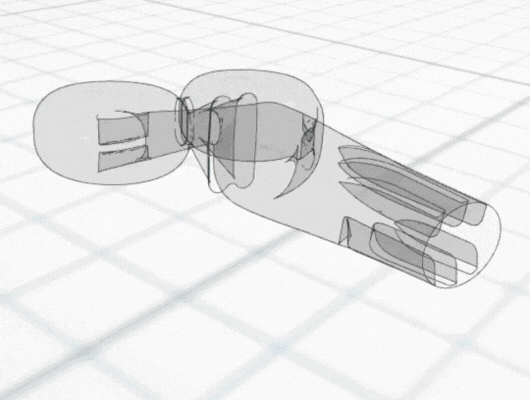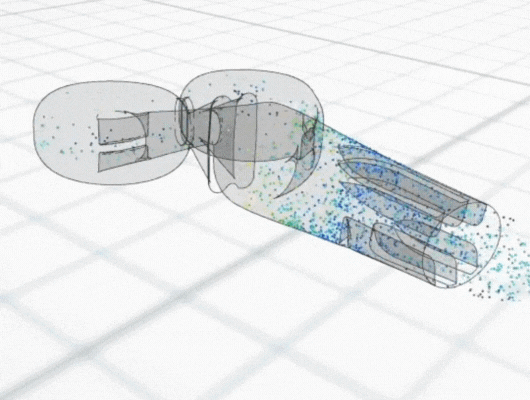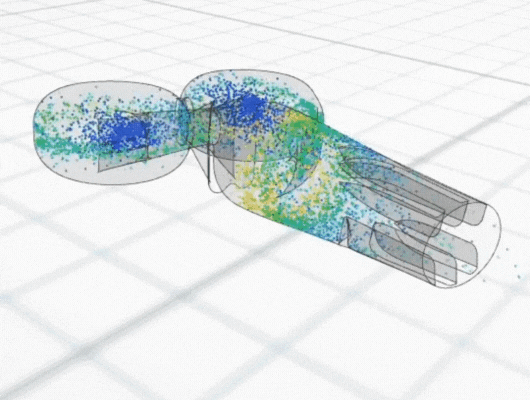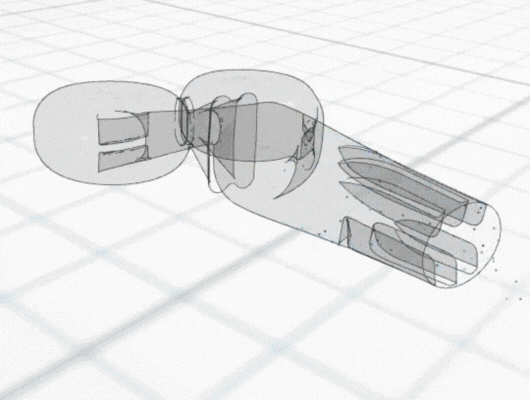
Mathematical models also often reveal patterns, relationships and dependencies that might not be immediately apparent. Through visual representations, such as graphs, charts and simulations, models can also help to visualise different aspects of the product and its technical feasibility.
When developing a math model, it is important to consider the level of complexity required. If the model is too simple, then the absence of unconsidered variables can lead to inaccurate results. If it’s too complex, the model can become hard to use. A good model should only be as complex as is necessary to achieve reliable results.
Once you have created a math model to represent the system and refined the model to target specific outputs, it’s important to move quickly into the ‘prototype and test’ iterative cycle to validate the theoretical understanding of the system.
Realising the demonstrator
Having understood the system and with an informed design schematic, industry standard Computer Aided Design (CAD) software can be used to realise the design in three dimensions. Once the final design is complete, the 3D CAD model also provides precise measurements and specifications that act as a blueprint for the prototyping process. This data can be directly used to create physical prototypes through 3D printing or alternative manufacturing processes.
Rapid prototyping such as this allows for quick and cost-effective production of physical prototypes that closely resemble the digital design. What’s more, feedback from functional testing and user evaluation can quickly be incorporated into the 3D CAD model to refine the design as well.
Overall, the iterative process of updating the CAD model and producing new prototypes helps optimise the product’s performance, appearance and usability.