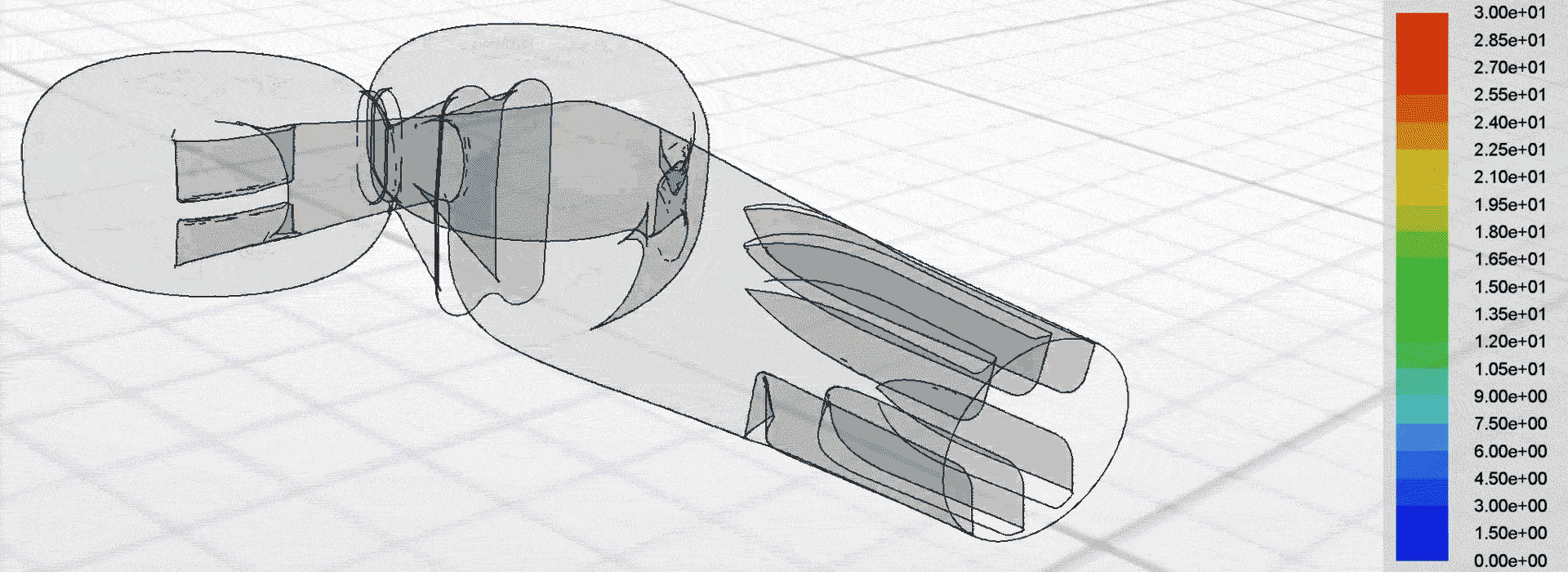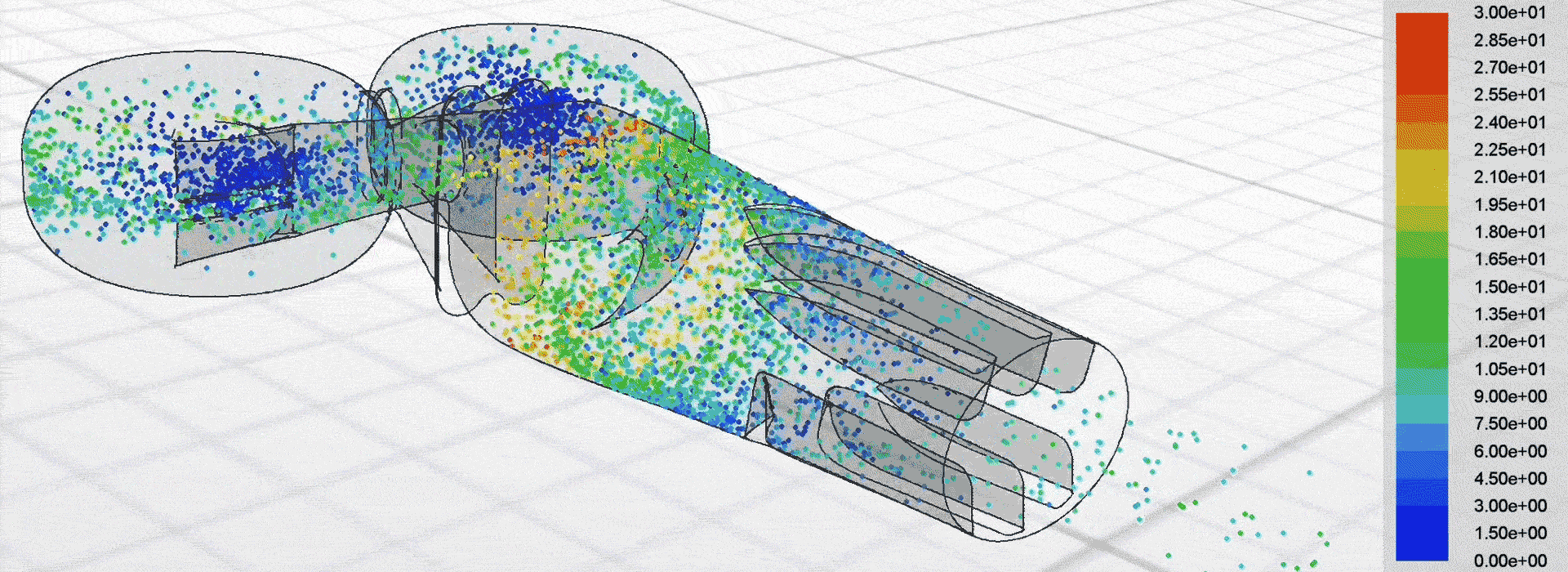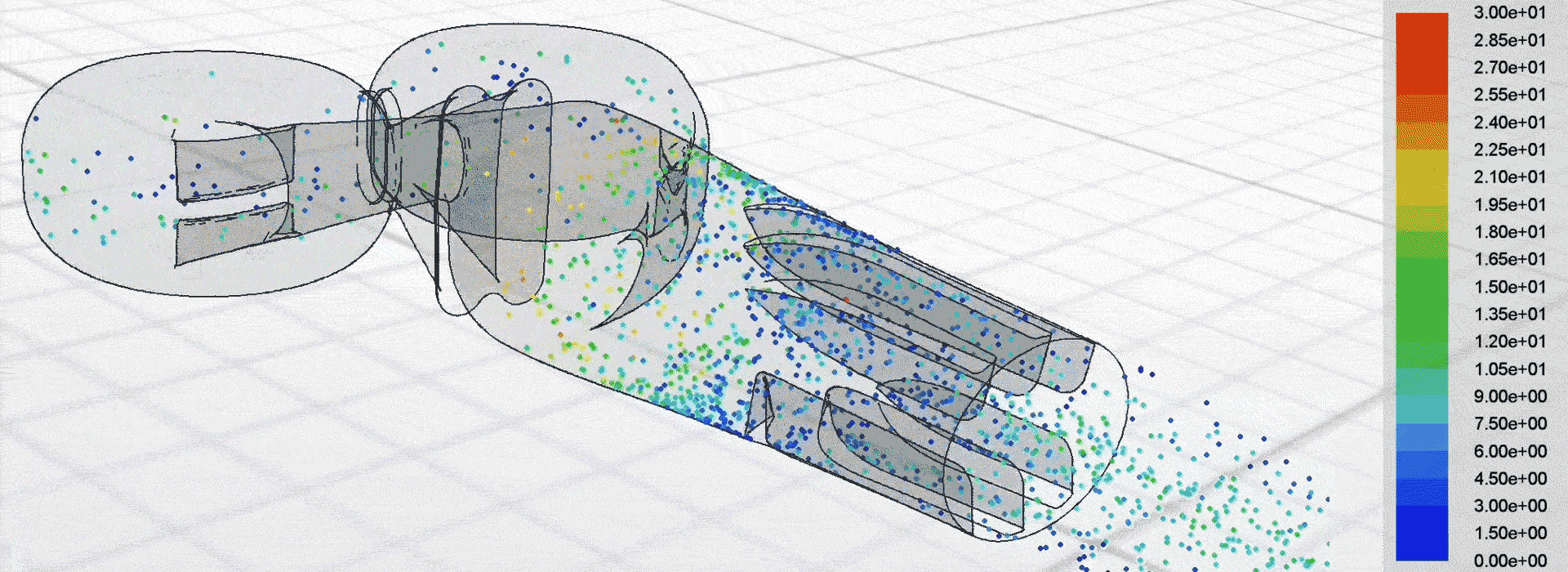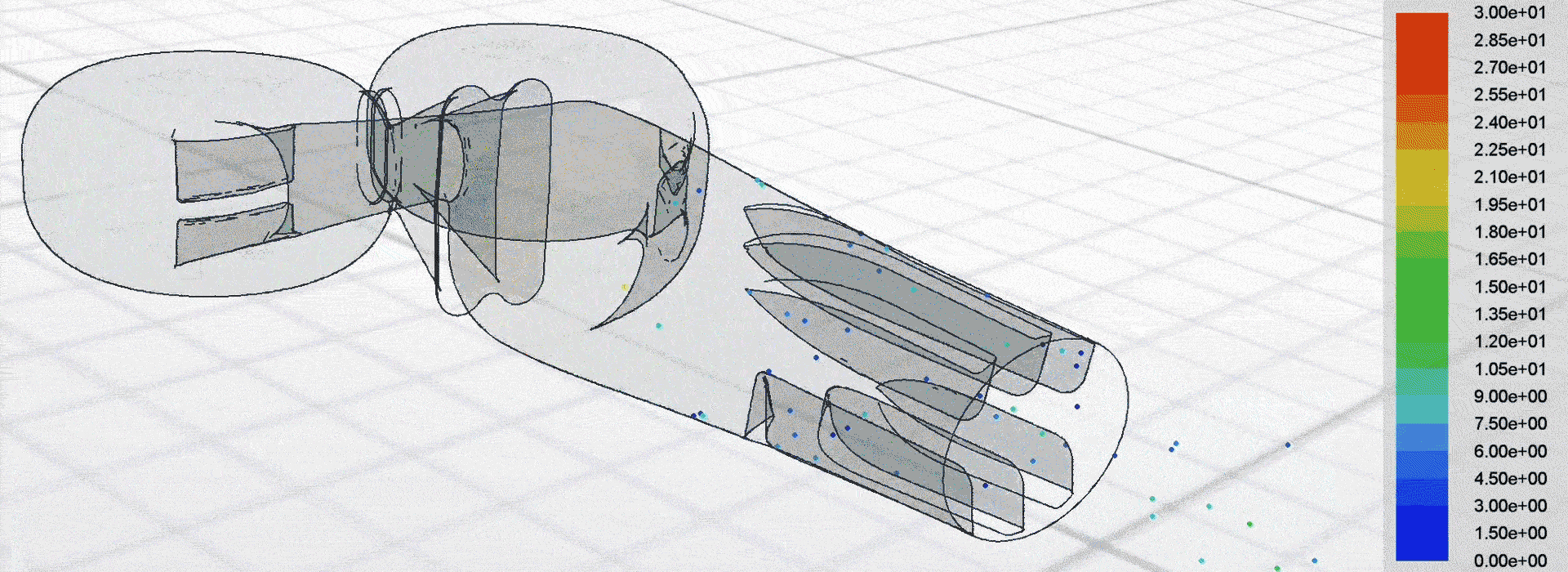
Airflow simulation for inhaler design using CFD
Challenge
Optimise the performance of a capsule dry powder inhaler (DPI) to increase fine particle drug delivery to the deep lung and in turn, improve the reliability of therapeutic outcomes for patients.
Approach
We used computational fluid dynamics (CFD) to simulate the complex airflow and powder dispersion within the DPI. This allowed us to inform key design changes for next-generation prototypes to be tested in the lab.
Outcome
Patients can receive a more consistent dose regardless of how strongly they inhale, which helps to manage their condition more effectively.