Is the future of vaccines intranasal?
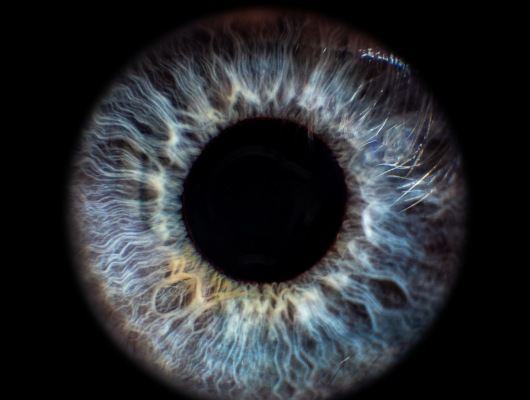
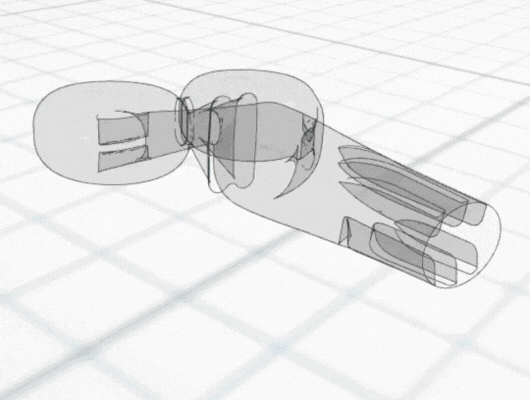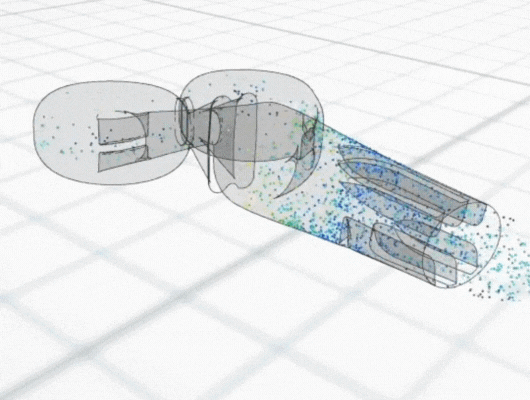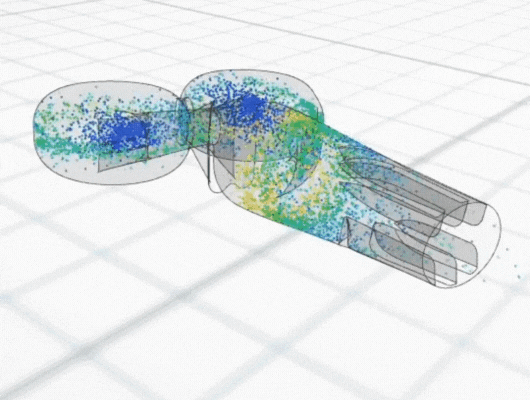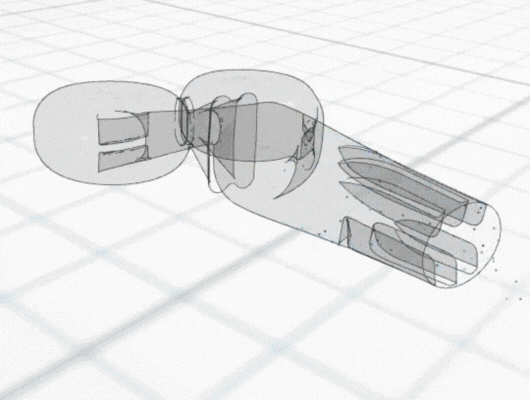
In recent years there has been considerable interest in developing intranasal COVID vaccines, with several in clinical development globally and a small number approved in countries such as India and Russia. New data has shown the potential for better efficacy with an intranasally-administered COVID vaccine, compared with the standard delivery route of an intra-muscular injected vaccine. This is perhaps not surprising, considering that COVID is a respiratory tract infection, and intranasal vaccines provide a targeted local delivery to the upper respiratory airway mucosa. Building immunity directly in the nasal mucosa also has better potential to prevent disease transmission, since the nasopharynx is the body’s first encounter with infectious aerosols. Early clinical data for intranasal vaccines suggest they avoid side-effects such as “flu symptoms” associated with existing COVID vaccines.
This growth in interest for intranasal vaccine delivery follows a trend of steady development of intranasal flu vaccines. One of those, FluMist/Fluenz Tetra, has been available to patients in US and EU markets for several years now. If a COVID intranasal vaccine is successful in reducing transmission rates, where the current intramuscular vaccines cannot, then this may increasingly become the delivery route of choice for vaccine developers, particularly for respiratory tract infections.
Intranasal delivery is also a growing route of interest for systemic drug delivery to the bloodstream for several medicines. Drugs such as analgesics and treatments for migraine, osteoporosis and sexual dysfunction, are all now established as intranasal therapies. Most recently, anti-anxiety/depression medication and opiate overdose reversal have also joined them. Currently, intranasal delivery is an important area of research for delivery of biological large molecules such as peptides or mRNA, because it bypasses the gastrointestinal route and the potential for digestive destruction of these sensitive compounds.
Contents