|
M. Mofidfar, B. Abdi, S. Ahadian, E. Mostafavi, T. A. Desai, F. Abbasi, Y. Sun, E. E. Manche, C. N. Ta and C. W. Flowers, “Drug delivery to the anterior segment of the eye: A review of current and future treatment strategies,” International Journal of Pharmaceutics, 2021. |
|
I. Rykowska, I. Nowak and N. Rafal, “Soft Contact Lenses as Drug Delivery Systems: A Review,” Molecules, 2021. |
|
R. Saabye and M. Jacobsen, “MISTGO – A New Microdosing Eye Medication Delivery System,” ONdrugDelivery, 11 March 2023. [Online]. Available: https://www.ondrugdelivery.com/mistgo-a-new-microdosing-eye-medication-delivery-system/. |
|
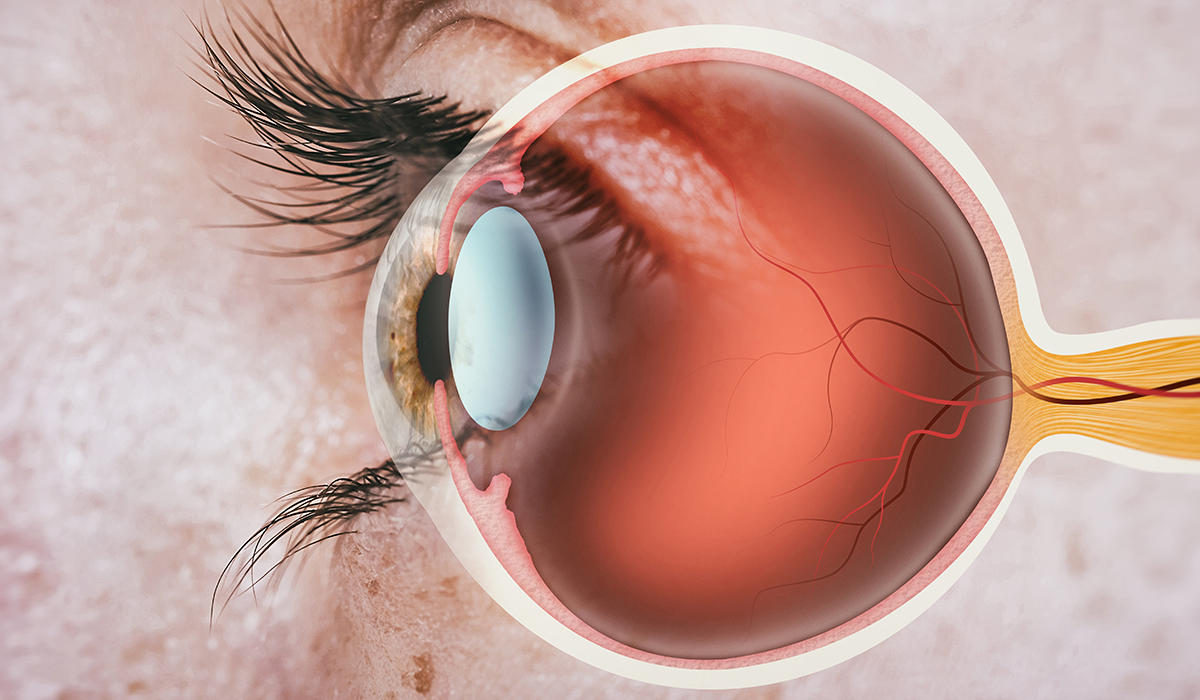
Editorial Team from Chronic Dry Eye, “Chronic Dry Eye: Parts and Function of Your Eyes and Tears,” Chronic Dry Eye, 19 April 2021. [Online]. Available: https://chronicdryeye.net/tears-function. |
|
S. Marx, J. Eckstein and W. Sickenberger, “Objective Analysis of Pre-Lens Tear Film Stability of Daily Disposable Contact Lenses Using Ring Mire Projection,” Objective Analysis of Pre-Lens Tear Film Stability of Daily Disposable Contact Lenses Using Ring Mire Projection, vol. 12, pp. 203-211, 2020. |
|
E. Mullin, “The First Drug-Releasing Contact Lens Is Here,” Wired, 30 March 2022. [Online]. Available: https://www.wired.com/story/the-first-drug-releasing-contact-lens-is-here/. |
|
“Johnson & Johnson Vision’s Investigational Antihistamine-Releasing Contact Lens Demonstrates Positive Phase 3 Results,” Johnson & Johnson, 26 March 2019. [Online]. Available: https://www.jjvision.com/press-release/johnson-johnson-visions-investigational-antihistamine-releasing-contact-lens. |
|
“Johnson & Johnson Vision Care Receives FDA Approval for ACUVUE® Theravision™ with Ketotifen – World’s First and Only Drug-Eluting Contact Lens,” Johnson & Johnson, 2 March 2022. [Online]. Available: https://www.jjvision.com/press-release/johnson-johnson-vision-care-receives-fda-approval-acuvuer-theravisiontm-ketotifen. |
|
B. Pall, Interviewee, First drug-eluting contact lens approved in Japan. [Interview]. 15 April 2021. |
|
“Contact Lens Drug Delivery Platform,” MediPrint Ophthalmics, [Online]. Available: https://mediprintlens.com/technology-science/. |
|
M. Barnett, “Contact Lens Drug Delivery Moves Closer to Reality,” [Online]. Available: https://na-prod-aventri-files.s3.amazonaws.com/html_file_uploads/2d5f8c7b3392bc6562add24a21eaad9e_Barnett_GSLS_poster_FINAL_1.7.2022.pdf?response-content-disposition=inline%3Bfilename%3D%22Barnett_GSLS_poster_FINAL_1.7.2022.pdf%22&response-content-type=ap. |
|
Study of LL-BMT1 in Patients With Elevated Intraocular Pressure, 2021. |
|
Therapeutic Contact Lens Drug Delivery System (TCL-DDS) in Patients With Recurrent Cystoid Macular Edema (ContactLens), 2020. |
|
Latanoprost Eluting Contact Lens for Treating Glaucoma and Ocular Hypertension, 2020. |
|
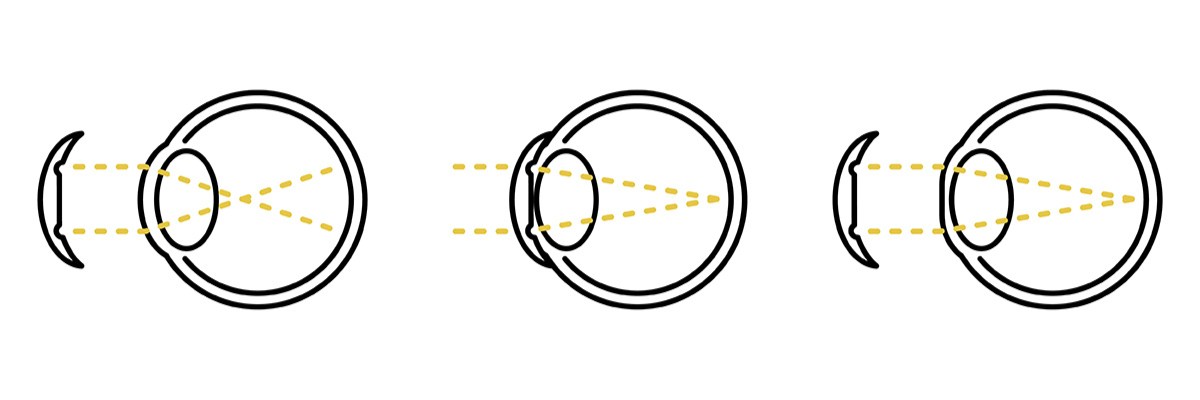
“Nearsightedness,” Mayo Clinic, [Online]. Available: https://www.mayoclinic.org/diseases-conditions/nearsightedness/symptoms-causes/syc-20375556#:~:text=Nearsightedness%20(myopia)%20is%20a%20common,to%20bend%20(refract)%20inaccurately. |
|
B. A. Holden, T. R. Fricke, D. A. Wilson, M. Jong, K. S. Naidoo, P. Sankarifurg, T. Y. Wong, T. K. Naduvilath and S. Resnikoff, “Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050,” Ophthalmology, vol. 123, no. 5, pp. 1036-1042, 2016. |
|
J. S. Harthan, “Overnight Orthokeratology for Myopia: What Does the Evidence Say?,” Review of Myopia Management, 3 June 2019. [Online]. Available: https://reviewofmm.com/overnight-orthokeratology-for-myopia-management-what-does-the-evidence-say/. |
|
NHS, “Glaucoma,” NHS, [Online]. Available: https://www.nhs.uk/conditions/glaucoma/#:~:text=Glaucoma%20is%20a%20common%20eye,not%20diagnosed%20and%20treated%20early. |
|
NHS, “Making a decision about Open-Angle Glaucoma,” [Online]. Available: https://www.england.nhs.uk/wp-content/uploads/2022/07/Making-a-decision-about-open-angle-glaucoma.pdf. |
|
C. Kent, “Treating with SLT First: The Pros and Cons,” Review of Ophthalmology, 9 June 2017. [Online]. Available: https://www.reviewofophthalmology.com/article/treating-with-slt-first-the-pros-and-cons. |
|
“Startup Developing New Glaucoma Treatment,” Inside INdiana Business, 14 November 2021. [Online]. Available: https://www.insideindianabusiness.com/videos/startup-developing-new-glaucoma-treatment. |
|
Purdue Research Foundation, “Purdue-affiliated startup developing non-invasive, effective contact lenses and glasses to treat glaucoma, prevent blindness,” Purdue Research Foundation, 31 May 2017. [Online]. Available: https://www.purdue.edu/newsroom/releases/2017/Q2/purdue-affiliated-startup-developing-non-invasive,-effective-contact-lenses-and-glasses-to-treat-glaucoma,-prevent-blindness.html. |
|
“Bionode: Jane Fischer — Global Innovations,” 2021. [Online]. Available: https://vimeo.com/502323679. |
|
Fda, “FDA permits marketing of device that senses optimal time to check patient’s eye pressure,” FDA, 4 March 2016. [Online]. Available: https://www.fda.gov/news-events/press-announcements/fda-permits-marketing-device-senses-optimal-time-check-patients-eye-pressure. |
|
X. Ma, S. Ahadian, S. Liu, J. Zhang and S. Liu, “Smart Contact Lenses for Biosensing Applications,” Advanced Intelligent Systems, vol. 3, no. 5, 2021. |
|
Photonics.com, “Diabetic Smart Contact Lenses Developed by South Korean Research Team,” Photonics Media, 15 January 2020. [Online]. Available: https://www.photonics.com/Articles/Diabetic_Smart_Contact_Lenses_Developed_by_South/a65468. |
|
“Importance of Proper Contact Lens Fitting,” Aspire Vision Care, [Online]. Available: https://www.aspirevisioncare.com/eyeglasses-contacts/contact-lenses/importance-of-proper-contact-lens-fitting/. |
|
“The EyePrint Impression Process: Easy, Painless, Precise.,” EyePrint Prosthetics, [Online]. Available: https://eyeprintpro.com/process/. |
|
H. Mirzajani, H. Mirlou, E. Istif, R. Singh and L. Beker, “Powering smart contact lenses for continuous health monitoring: Recent advancements and future challenges,” Biosensors and Bioelectronics, 2021. |
|
J. Park, J. Kim, S.-Y. Kim, W. H. Cheong and J. Jang, “Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays,” Science Advances, vol. 4, no. 1, 2018. |