Transforming Point of Care diagnostics
Challenge
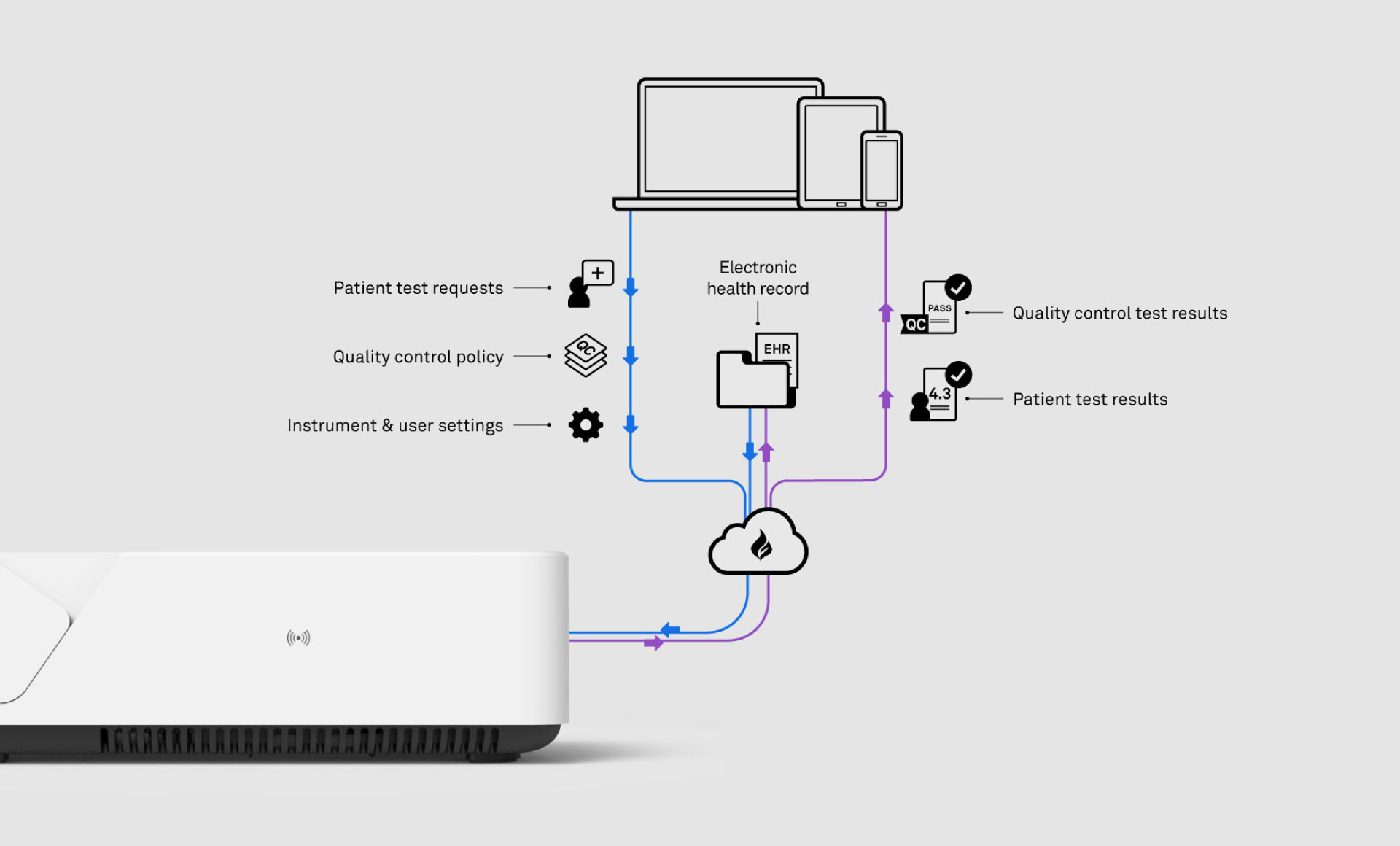
To develop a hand-held, Point of Care (PoC) instrument which could run fluorometric, multiplex immunoassays using microfluidic strips. The instrument also had to deliver an outstanding user experience – comparable to the best consumer electronics – along with fully secure cloud connectivity to electronic health records.
Approach
With LumiraDx, we formed a multi-disciplinary team which didn’t just include engineers, designers and physicists, but also biochemists, digital design experts and human factors specialists. The skills and experience of the group at Team Consulting complimented the client’s experience and knowledge, particularly in assay development, consumables and instrumentation. The teams worked closely together iterating quickly and efficiently to prove all the aspects of the system from the embedded software and microfluidic control, to the product usability and integration with electronic medical records.
Outcome
The LumiraDX platform was developed, tested, CE marked and transferred to manufacture in less than three years.