Wearable injection device for continuous drug delivery
Challenge
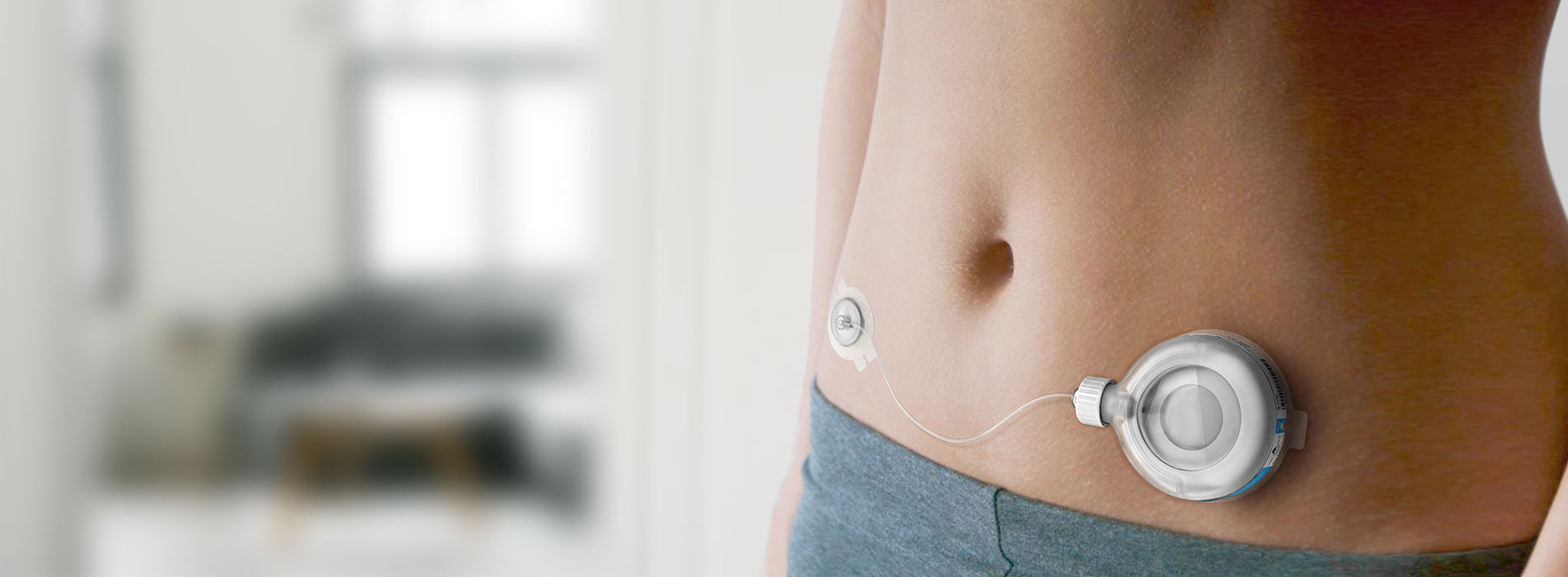
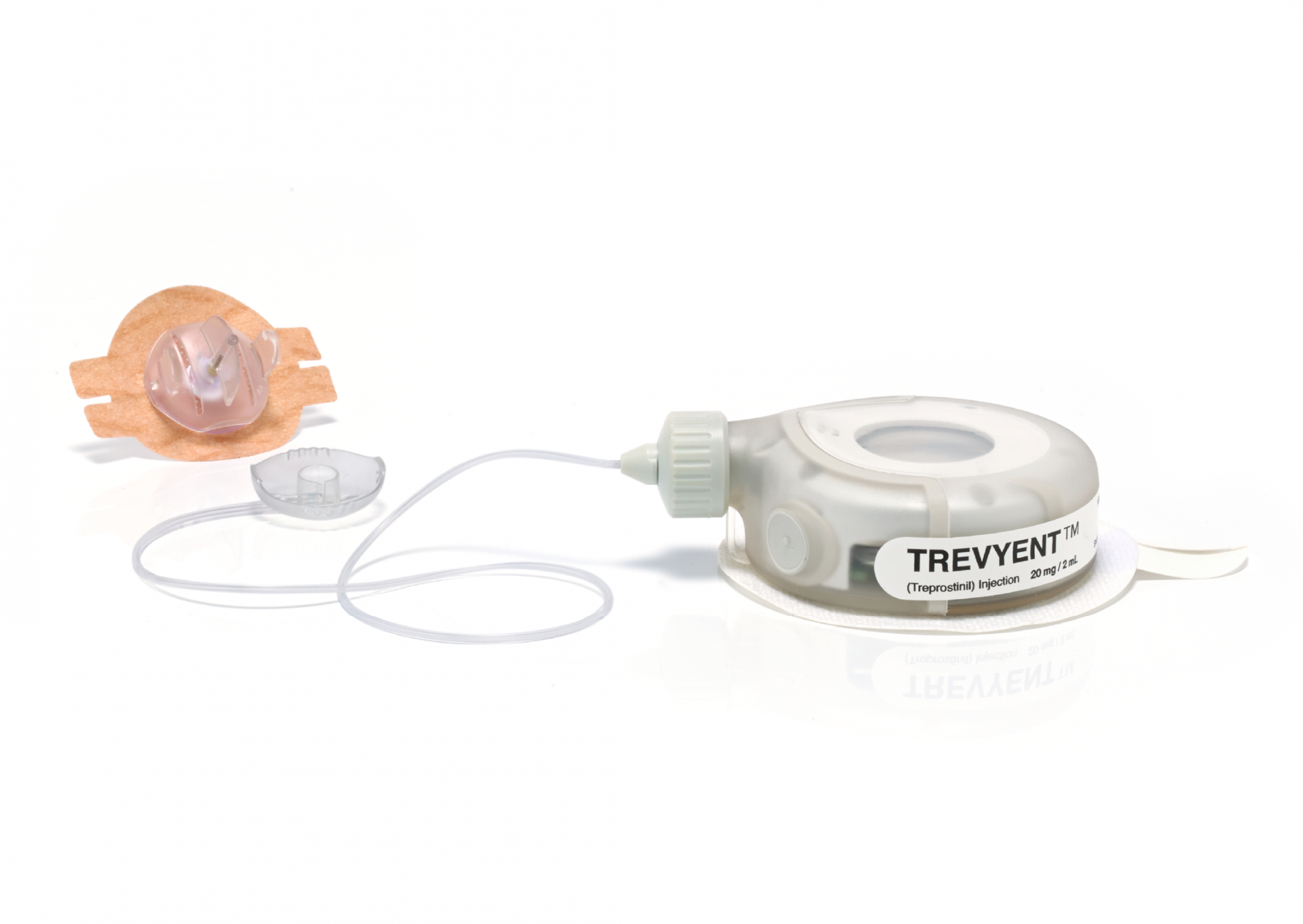
Take a wearable injector platform from proof-of-principle to FDA approval. The product had to be capable of delivering 2ml of Treprostinil drug over a 48-hour period with a high degree of accuracy, control and safety over a broad range of conditions.
Approach
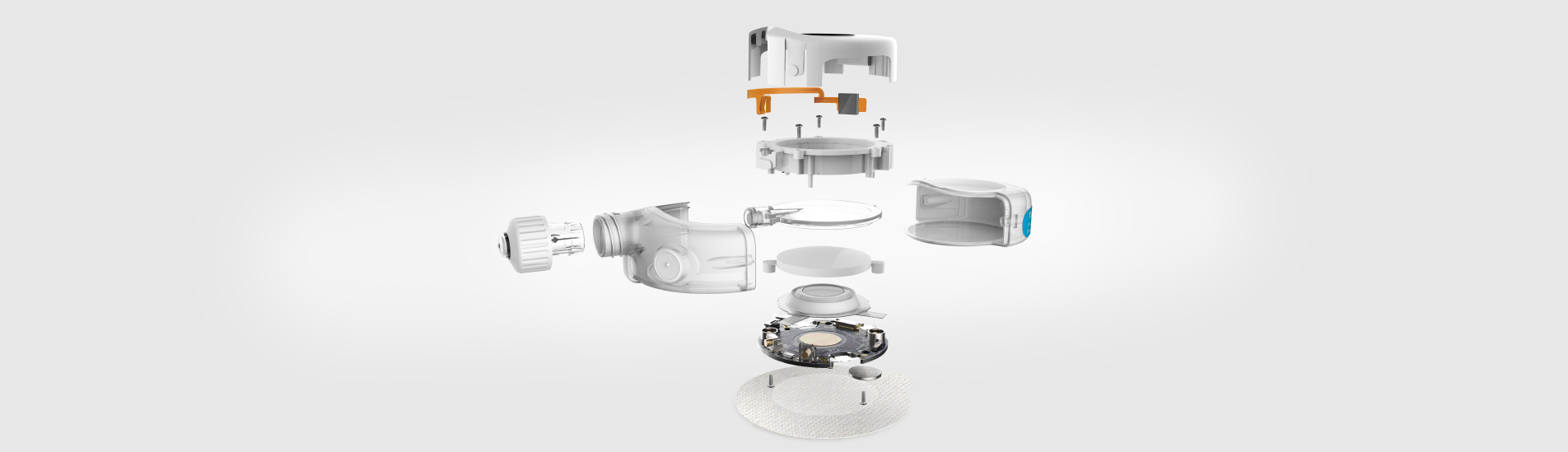
Using our broad skills in electronics, mechanical engineering, software, design and human factors, Team Consulting harnessed a novel technology and met requirements of a precision drug delivery device. We also supported SteadyMed through the submission of a new drug application (NDA) to the FDA. As a company in the early stages of development, our client needed a partner who was flexible, cooperative and accommodating. As SteadyMed’s partner, it was also important to understand the pressures of a young company from investors, timelines and strict budgets.
Outcome
The final product is now ready for volume manufacture, with a complete Design History File.