Development process
During the project, we designed, optimized and verified a number of improved and novel components and mechanisms, utilizing a range of analytical and empirical engineering tools and techniques. As a result, we:
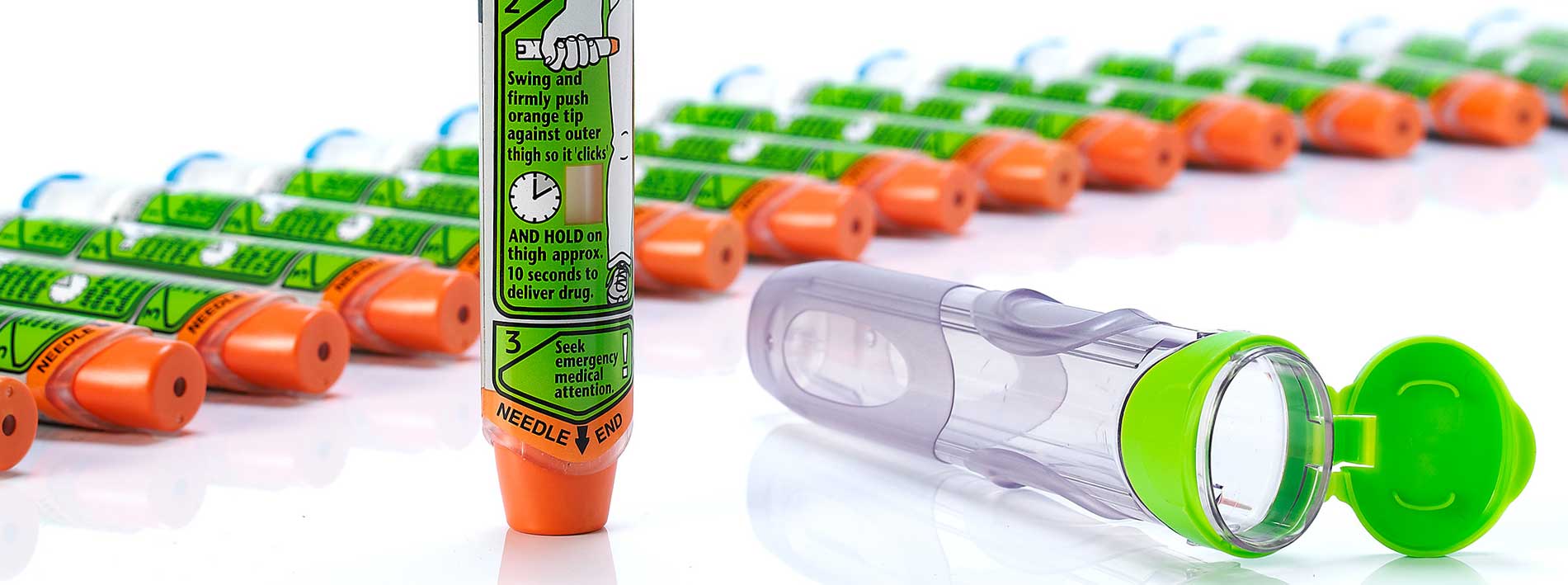
- developed a high-volume, platform product offering the opportunity to accommodate a range of drug container and needle sizes, while leaving the existing primary pack technology unchanged
- reduced the magnitude and variability of activation force, while also reducing production costs
- integrated an automatic needle protection system while minimizing the impact on device size
- delivered a device technology which resulted in new intellectual property for our client
Following pilot scale design verification, we worked very closely with Meridian’s manufacturing partners and suppliers to support scale-up to high-volume manufacture of the device, including validation of production tooling and the development, build and commissioning of automated assembly equipment. Finally, we provided a comprehensive handover of design history and technical file documentation including CAD database and risk management file.
As part of the project, we also developed, in parallel, a low-cost training aid. This trainer is recognizable as an EpiPen but distinguishable from the active drug-filled product, allows repeated reuse and features a minimal number of components.