Measuring the carbon footprint of drug delivery devices with LCA
Challenge
Assess the environmental impact of a range of drug delivery devices from a simple syringe to complex auto-injectors.
Approach
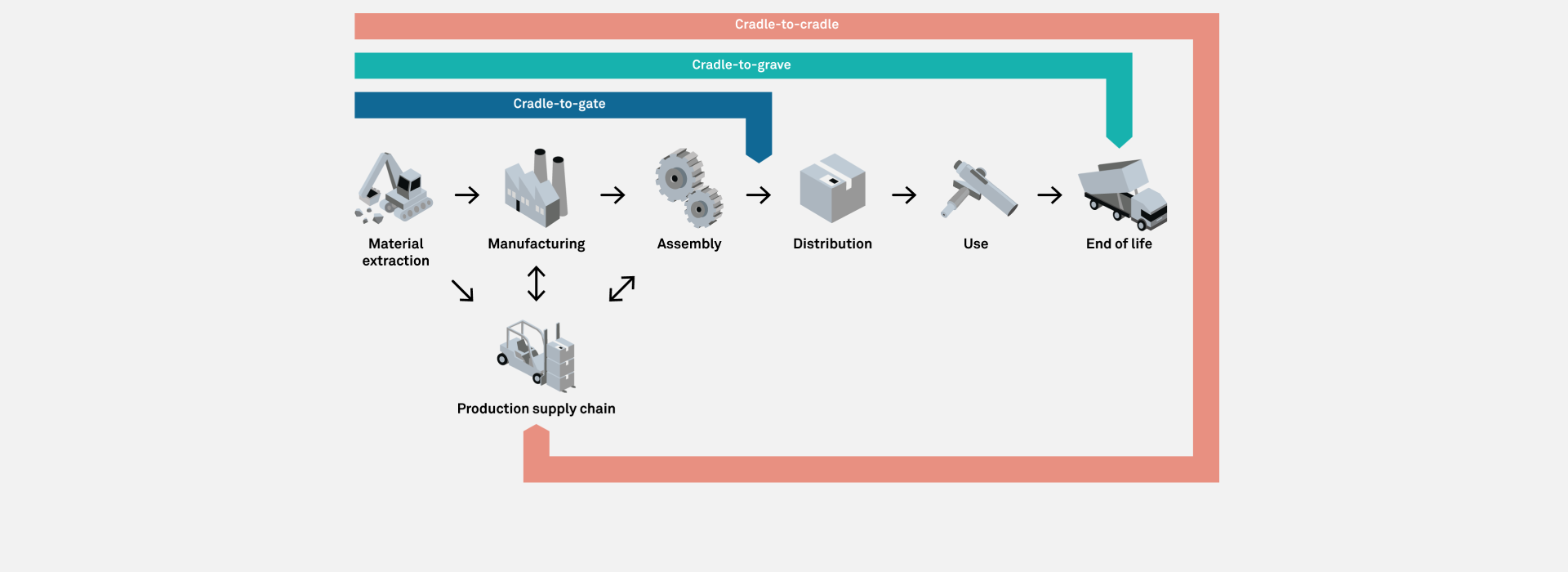
Our client, a pharmaceutical company, approached Team Consulting to evaluate the carbon footprint of six of their drug delivery devices. We conducted a systematic Life Cycle Analysis (LCA) of each device. To do this, we used data on the products and their supply chain in combination with carbon footprint statistics from LCA databases and literature.
Outcome
This analysis produced a robust estimation of carbon footprint between the “cradle” and “gate” stages (between material extraction and the factory gates, ahead of distribution). We highlighted factors that caused high emissions in all devices (the units of the analysis were in grams of carbon dioxide equivalent emissions, gCO2e). This allowed our client to make data-informed decisions to reduce their environmental impact in areas such as supply chain management. Our analysis also prompted them to modify their packaging elements to further reduce their carbon footprint.