Over the past few years, many pharma companies have chosen to deliver their therapies through ‘off-the-shelf’ platform devices rather than developing their own technology from the ground up. This approach offers huge savings in development costs, timescales and can reduce risk dramatically, but we are often asked the same questions about what we can do to improve the products.
How can we help make the product stand out from other brands who may be using the same platform? How can we make it feel like it’s from the same family as other products within the brand? How can we ensure we deliver a premium brand experience when we are so restricted in what we can change?
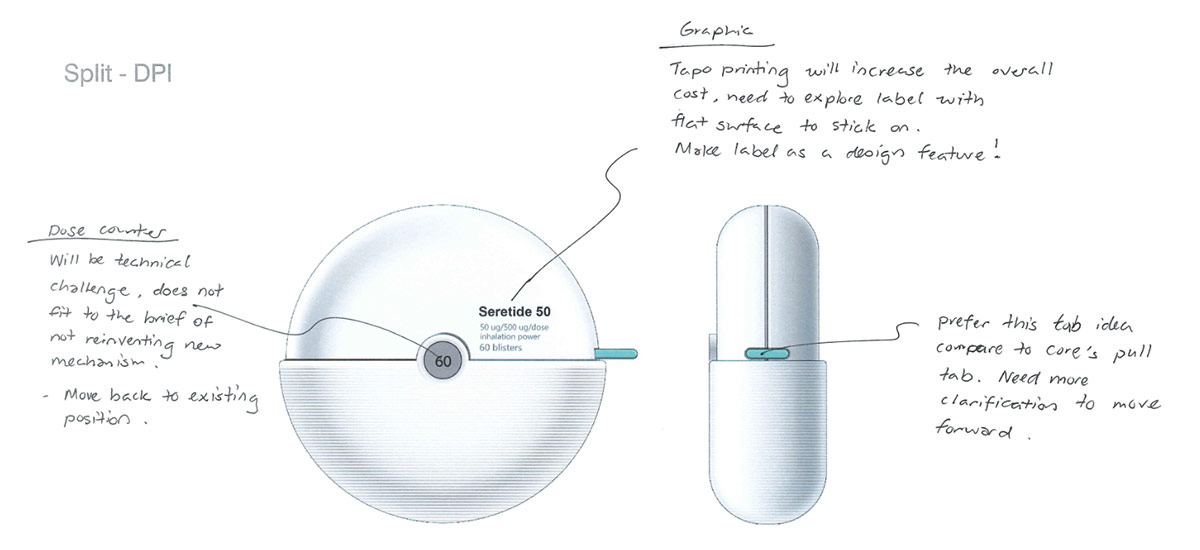
As medical device designers, we understand that achieving a coherent experience across a range of different devices can be difficult. This can seem especially challenging when working with previously developed platform products which may have been developed separately, often years apart, by different device companies. It’s a challenge we’ve faced many times, but by understanding the constraints and working creatively within them, a lot can be achieved.
Our brief was to create a family of three inhalers: a dry-powder inhaler (DPI), pressurized metered-dose inhaler (pMDI) and a unit dose capsule inhaler.
The devices needed to be distinctive, iconic and stand out from current devices in the market, whilst not changing the existing device architecture or core technologies of three approved inhaler platforms.
The products needed to demonstrate that the licensing company understood the emotional and practical needs of its customers, by creating inhalers that felt personal, approachable and would fit with its users’ lifestyles.