Controlling bleeds during surgery
Challenge
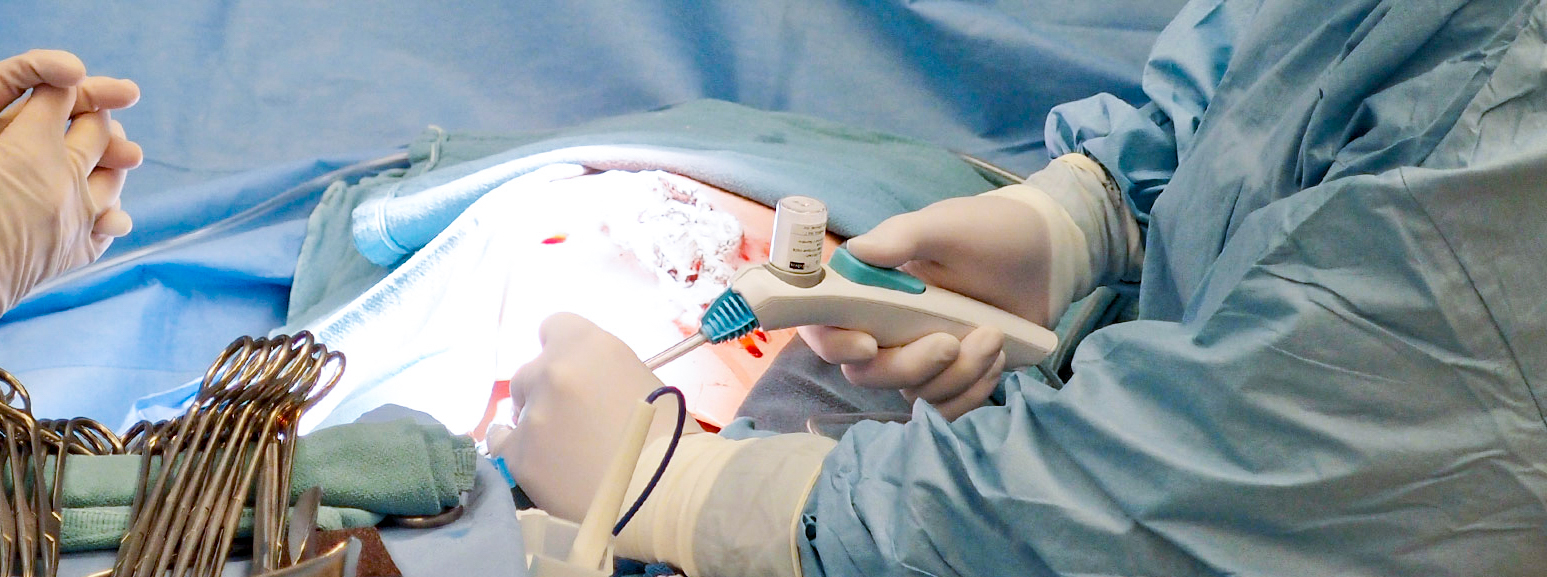
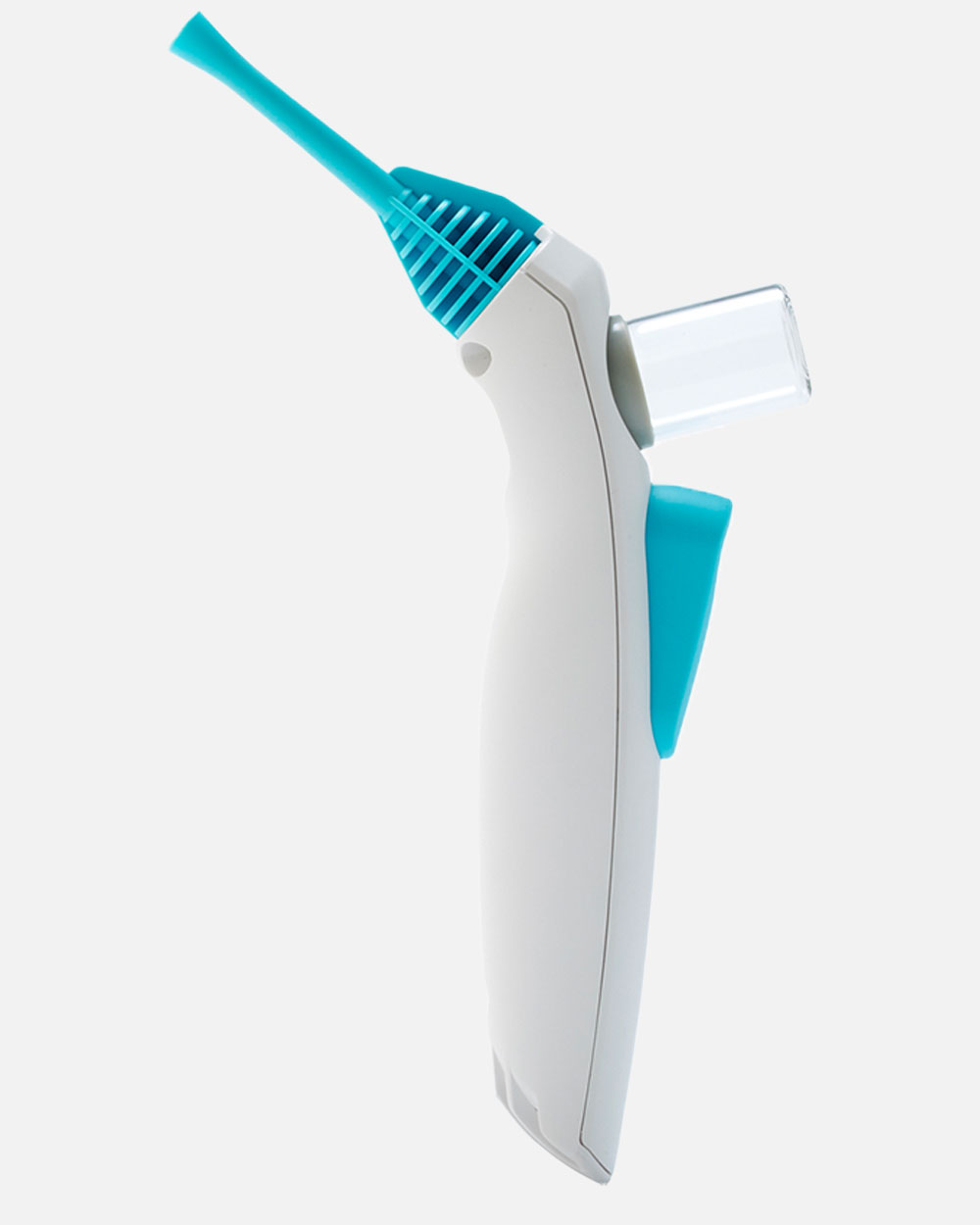
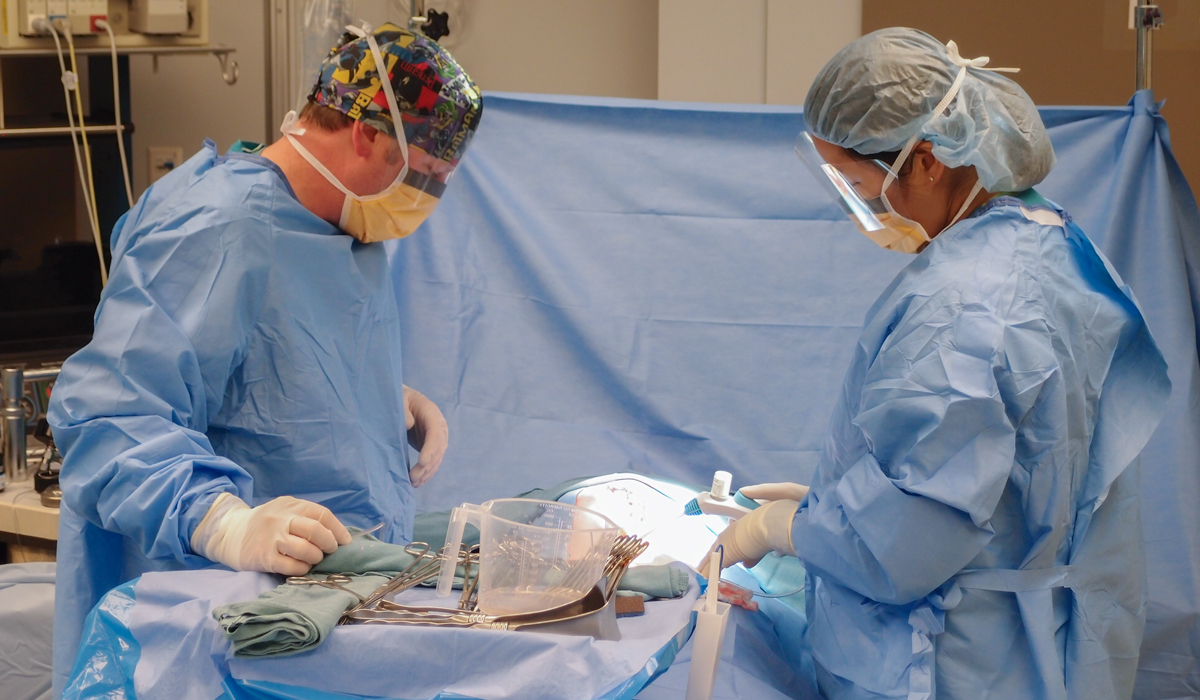
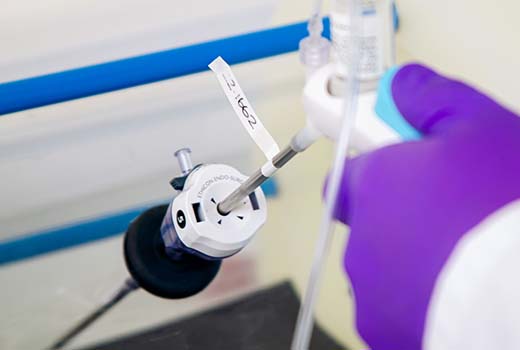
Supporting a start-up in the development of a device that could deliver a fine, sticky powder precisely to control bleeds during a variety of surgical procedures.
Approach
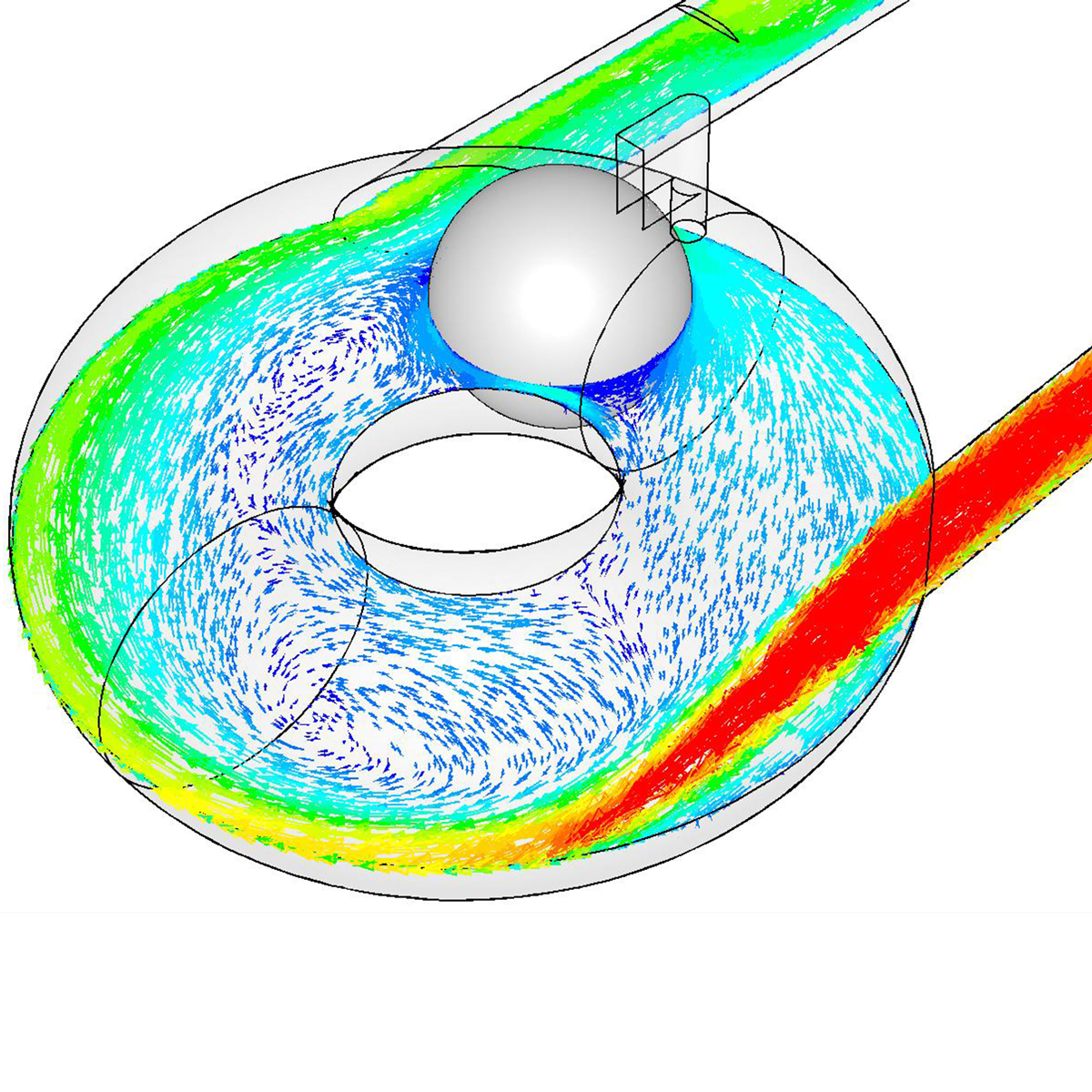
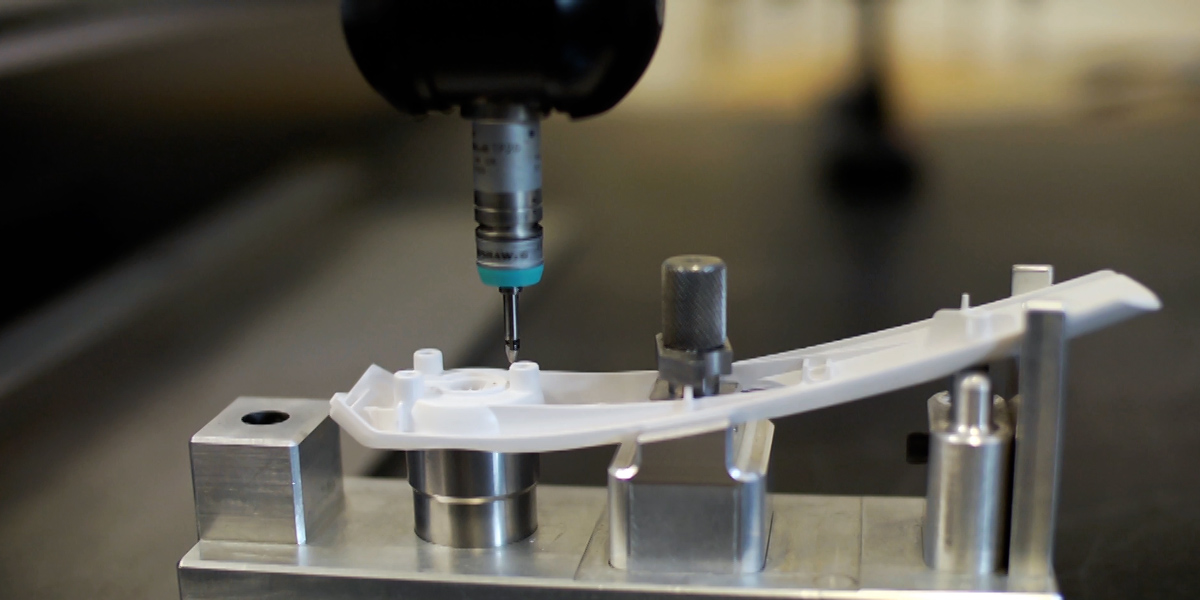
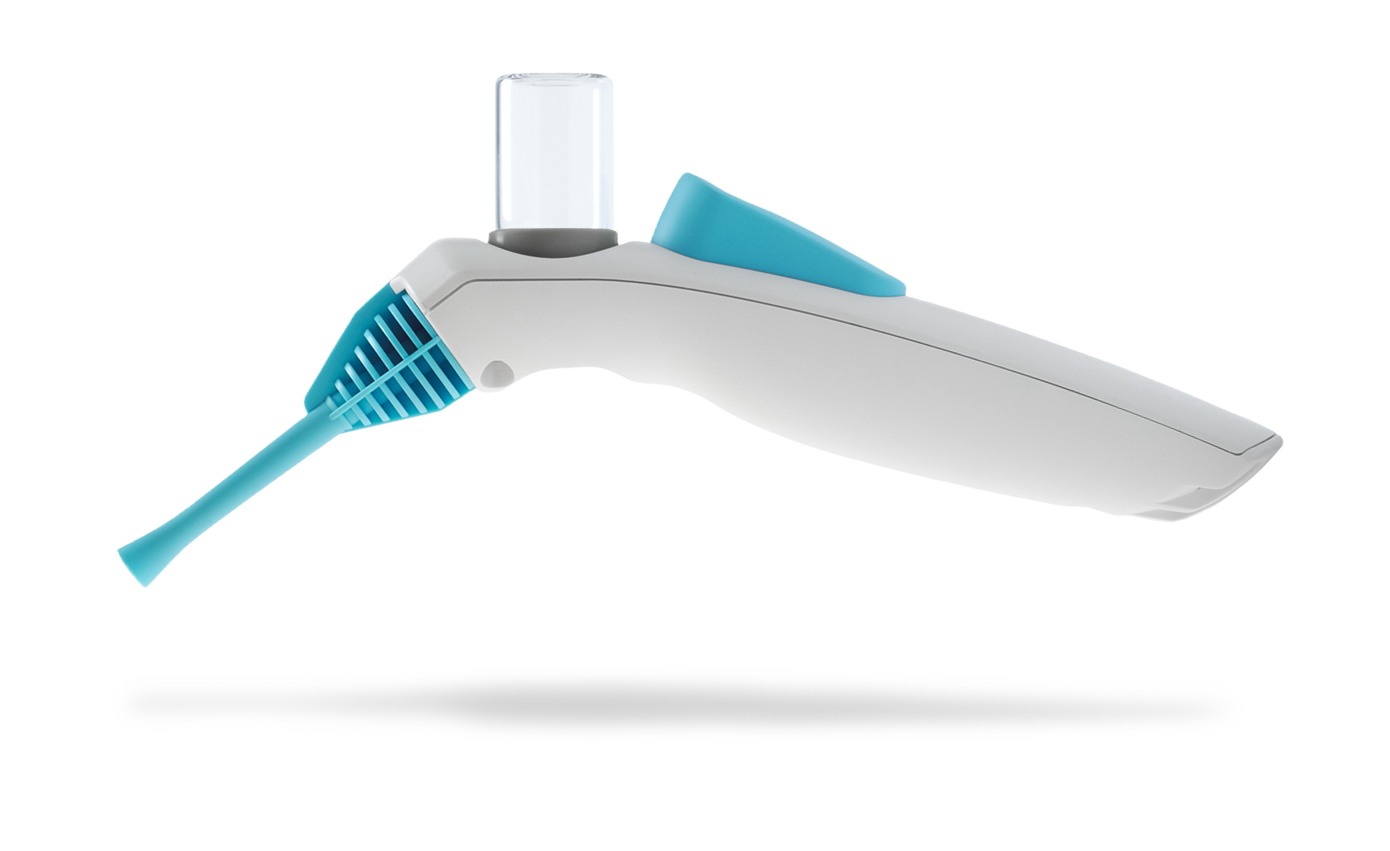
Following extensive research with leading surgeons in Europe and the United States, we developed a highly intuitive device for delivering a novel powder. Our remit included selection and management of a contract manufacturing organization for high-volume manufacture.
Outcome
We developed a groundbreaking surgical device which received regulatory approval in Europe and the United States, leading to the company being fully acquired for $240M.