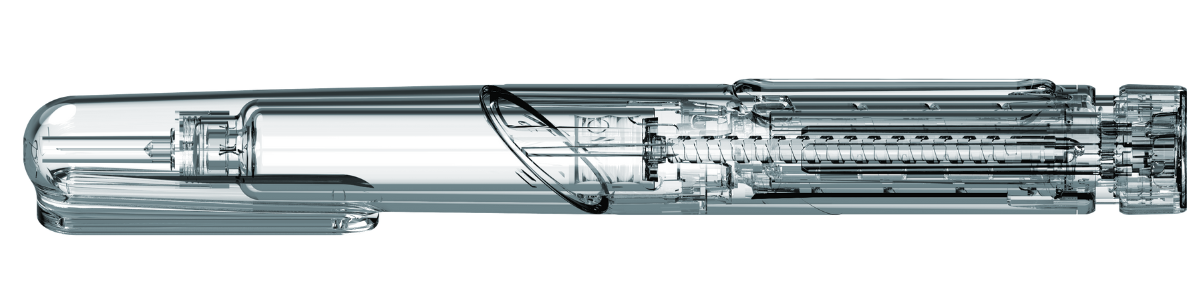
Development of an 80IU variable dose injection device
Challenge
Create a variable dose insulin pen that built on the client’s existing brand identity, and increased the maximum dosage capacity from 60IU to 80UI.
Approach
We worked closely with the client’s team to define the look and feel of the device based on user insights, as well as ensuring the device was robust and suitable for industrialisation.
Outcome
We produced an elegant, functional and cost-effective device solution for our client, as well as developing product packaging and IFU.